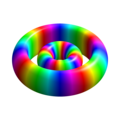
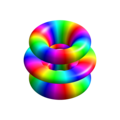
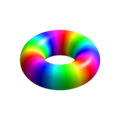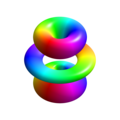氫軌域/物理
Single electron orbitals of the hydrogen atom which constitute simultaneous eigenstates of H, L² and Lz. Each orbital is aligned around the z-axis, but it remained a valid eigenfunction if rotated to any direction.
For real-valued orbitals, as convenient in chemistry, look at Hydrogen orbitals 3D real.
n = 1
-
n=1, l=0, m=0
-
n=1, l=0, m=0
-
n=1, l=0, m=0
n = 2
-
n=2, l=0, m=0
-
n=2, l=0, m=0
-
n=2, l=0, m=0
-
n=2, l=1, m=-1
-
n=2, l=1, m=0
-
n=2, l=1, m=1
n = 3
-
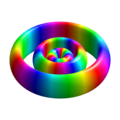
n=3, l=0, m=0
-
n=3, l=0, m=0
-
n=3, l=0, m=0
-
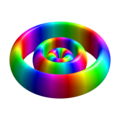
n=3, l=1, m=-1
-
n=3, l=1, m=0
-
n=3, l=1, m=1
-
n=3, l=2, m=-2
-
n=3, l=2, m=-1
-
n=3, l=2, m=0
-
n=3, l=2, m=1
-
n=3, l=2, m=2
n = 4
-
n=4, l=0, m=0
-
n=4, l=0, m=0
-
n=4, l=0, m=0
-
n=4, l=1, m=-1
-
n=4, l=1, m=0
-
n=4, l=1, m=1
-
n=4, l=2, m=-2
-
n=4, l=2, m=-1
-
n=4, l=2, m=0
-
n=4, l=2, m=1
-
n=4, l=2, m=2
-
n=4, l=3, m=-3
-
n=4, l=3, m=-2
-
n=4, l=3, m=-1
-
n=4, l=3, m=0
-
n=4, l=3, m=1
-
n=4, l=3, m=2
-
n=4, l=3, m=3
n = 5
-
n=5, l=0, m=0
-
n=5, l=0, m=0
-
n=5, l=0, m=0
-
n=5, l=1, m=-1
-
n=5, l=1, m=0
-
n=5, l=1, m=1
-
n=5, l=2, m=-2
-
n=5, l=2, m=-1
-
n=5, l=2, m=0
-
n=5, l=2, m=1
-
n=5, l=2, m=2
-
n=5, l=3, m=-3
-
n=5, l=3, m=-2
-
n=5, l=3, m=-1
-
n=5, l=3, m=0
-
n=5, l=3, m=1
-
n=5, l=3, m=2
-
n=5, l=3, m=3
-
n=5, l=4, m=-4
-
n=5, l=4, m=-3
-
n=5, l=4, m=-2
-
n=5, l=4, m=-1
-
n=5, l=4, m=0
-
n=5, l=4, m=1
-
n=5, l=4, m=2
-
n=5, l=4, m=3
-
n=5, l=4, m=4
n = 6
-
n=6, l=0, m=0
-
n=6, l=0, m=0
-
n=6, l=0, m=0
-
n=6, l=1, m=-1
-
n=6, l=1, m=0
-
n=6, l=1, m=1
-
n=6, l=2, m=-2
-
n=6, l=2, m=-1
-
n=6, l=2, m=0
-
n=6, l=2, m=1
-
n=6, l=2, m=2
-
n=6, l=3, m=-3
-
n=6, l=3, m=-2
-
n=6, l=3, m=-1
-
n=6, l=3, m=0
-
n=6, l=3, m=1
-
n=6, l=3, m=2
-
n=6, l=3, m=3
-
n=6, l=4, m=-4
-
n=6, l=4, m=-3
-
n=6, l=4, m=-2
-
n=6, l=4, m=-1
-
n=6, l=4, m=0
-
n=6, l=4, m=1
-
n=6, l=4, m=2
-
n=6, l=4, m=3
-
n=6, l=4, m=4
-
n=6, l=5, m=-5
-
n=6, l=5, m=-4
-
n=6, l=5, m=-3
-
n=6, l=5, m=-2
-
n=6, l=5, m=-1
-
n=6, l=5, m=0
-
n=6, l=5, m=1
-
n=6, l=5, m=2
-
n=6, l=5, m=3
-
n=6, l=5, m=4
-
n=6, l=5, m=5
Collections
-
orbitals with one radial node up to m=4
-
some orbitals with m≥0
-
m-eigenvalues and ±m superposition orbitals
-
All orbitals up to n=3
-
All orbitals up to n=4
-
Density Plots