氫軌域/化學
Single electron orbitals of the hydrogen atom. The wavefunctions are eigenfunctions of H and L² and are furthermore chosen to be real.
For complex eigenfunctions of Lz, as convenient in physics, look at Hydrogen orbitals 3D.
n = 1
-
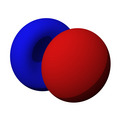
n=1, l=0, m=0
n = 2
-
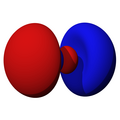
n=2, l=0, m=0
-
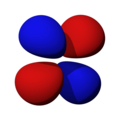
n=2, l=1, y
-
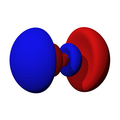
n=2, l=1, z
-
n=2, l=1, x
n = 3
-
n=3, l=0, m=0
-
n=3, l=1, y
-
n=3, l=1, z
-
n=3, l=1, x
-
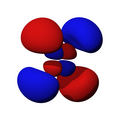
n=3, l=2, x²-y²
-
n=3, l=2, yz
-
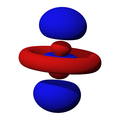
n=3, l=2, z²
-
n=3, l=2, xz
-
n=3, l=2, xy
n = 4
-
n=4, l=0, m=0
-
n=4, l=1, y
-
n=4, l=1, z
-
n=4, l=1, x
-
n=4, l=2, x²-y²
-
n=4, l=2, yz
-
n=4, l=2, z²
-
n=4, l=2, xz
-
n=4, l=2, xy
-
n=4, l=3, y(3x²-y²)
-
n=4, l=3, z(x²-y²)
-
n=4, l=3, yz²
-
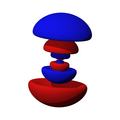
n=4, l=3, z³
-
n=4, l=3, xz²
-
n=4, l=3, xyz
-
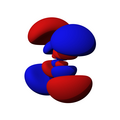
n=4, l=3, x(x²-3y²)
n = 5
-
n=5, l=0, m=0
-
n=5, l=1, y
-
n=5, l=1, z
-
n=5, l=1, x
-
n=5, l=2, x²-y²
-
n=5, l=2, yz
-
n=5, l=2, z²
-
n=5, l=2, xz
-
n=5, l=2, xy
...
n = 6
-
n=6, l=0, m=0
-
n=6, l=1, y
-
n=6, l=1, z
-
n=6, l=1, x
-
n=6, l=2, x²-y²
-
n=6, l=2, yz
-
n=6, l=2, z²
-
n=6, l=2, xz
-
n=6, l=2, xy
...
n = 7
-
n=7, l=0, m=0
...